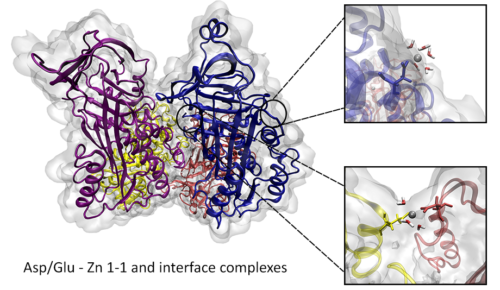
Paper “Interactions of zinc aqua complexes with ovalbumin at the forefront of the Zn2+/ZnO-OVO hybrid complex formation mechanism” published in Applied Surface Science. Experimental and theoretical approaches were used for a mechanistic study of the nanocomplexes formation through Zn2+(aq)-ovalbumin (OVA) binding. The determination of processes occurring at Zn2+(aq)-OVA interfaces and mechanism of hybrid complexes formation involved the modeling of kinetics and isotherm of Zn2+(aq) binding. Scanning electron microscopy, energy-dispersive X-ray analysis and X-ray diffraction were employed to characterize the nano-crystalline structure and morphology of the Zn2+/ZnO-OVO hybrid complex. Formation of hybrid complex was monitored using Fourier transform infrared spectroscopy. Specific Zn2+(aq) binding sites were pointed using MD simulations. Large-scale MD analysis was carried out to sample conformational space of the formed Zn2+(aq)-OVA complexes. Density functional theory (DFT) calculations were used to investigate the structures of the interaction complexes between zinc(II) and Asp−/Glu− and predict their respective IR spectra, and for mechanistic modeling of oxidation processes involved in the formation and stabilization Zn2+/ZnO-OVO hybrid complex. Negatively charged ovalbumin surface was locally neutralized through rapid Zn2+(aq) adsorption and formation of monolayers via 1:1 Asp−/Glu−/-Zn2+(aq) complexes. The ovalbumin monomer species began to agglomerate, stabilized with Asp−/Glu−-Zn2+(aq)-Glu−/Asp− interface complexes that promoted the formation of nanocomplexes. Finally, antimicrobial assays have shown that Zn2+/ZnO-OVO hybrid complex exhibit an inhibition effect against bacteria (A. baumanii, K. pneumonia) and yeast (C. albicans) strains.